Buffer Solutions: Understanding Their Functionality
This file provides comprehensive insights into buffer solutions, including their preparation and pH resistance mechanisms. Ideal for chemistry students and professionals seeking to understand buffer systems. Practice problems included for hands-on learning.
Edit, Download, and Sign the Buffer Solutions: Understanding Their Functionality
Form
eSign
Add Annotation
Share Form
How do I fill this out?
Begin by reading the instructions provided carefully. Next, identify the correct components needed to create a buffer solution. Finally, follow the practice problems to test your understanding.
How to fill out the Buffer Solutions: Understanding Their Functionality?
1
Read the instructions carefully.
2
Gather the necessary materials for buffer preparation.
3
Follow the demonstrated methods to create a buffer.
4
Complete the practice problems to reinforce learning.
5
Review your answers and concepts as needed.
Who needs the Buffer Solutions: Understanding Their Functionality?
1
Chemistry students who require practical knowledge about buffer solutions.
2
Teachers seeking resources for their chemistry classes.
3
Laboratory technicians who need to understand buffer preparation techniques.
4
Researchers studying acid-base reactions and their applications.
5
Anyone interested in chemistry concepts related to pH stability.
How PrintFriendly Works
At PrintFriendly.com, you can edit, sign, share, and download the Buffer Solutions: Understanding Their Functionality along with hundreds of thousands of other documents. Our platform helps you seamlessly edit PDFs and other documents online. You can edit our large library of pre-existing files and upload your own documents. Managing PDFs has never been easier.
Edit your Buffer Solutions: Understanding Their Functionality online.
Editing this PDF on PrintFriendly is easy and intuitive. Simply open the PDF in our editor and use the tools provided to make your changes. Once you're satisfied with your edits, you can download the updated PDF for your records.
Add your legally-binding signature.
To sign this PDF on PrintFriendly, navigate to the signature section within our editor. Use the provided tools to create your signature, and place it where needed. After signing, download your signed PDF for your documents.
Share your form instantly.
Sharing your PDF on PrintFriendly is seamless and straightforward. Once your document is ready, use the share functionality to distribute it to your audience. You can easily share through email or social media directly from our platform.
How do I edit the Buffer Solutions: Understanding Their Functionality online?
Editing this PDF on PrintFriendly is easy and intuitive. Simply open the PDF in our editor and use the tools provided to make your changes. Once you're satisfied with your edits, you can download the updated PDF for your records.
1
Open the PDF in PrintFriendly's editing interface.
2
Select the text or elements you wish to change.
3
Utilize editing tools to make your desired adjustments.
4
Review your edits to ensure accuracy.
5
Download the final version of your edited PDF.

What are the instructions for submitting this form?
To submit this form, please email your completed document to submissions@printfriendly.com. Alternatively, you can fax it to (123) 456-7890. Ensure that all required fields are filled out accurately to facilitate processing.
What are the important dates for this form in 2024 and 2025?
Important dates related to buffer solution applications will vary by institution and context. Typically, educational institutions may have deadlines for lab reports or coursework involving such materials. Always consult your course syllabus for specific dates.

What is the purpose of this form?
This form serves to educate users on the essential nature of buffer solutions and their preparation. By filling it out, users will gain insight into pH stability and the chemical reactions involved. It also provides practice problems to solidify understanding.

Tell me about this form and its components and fields line-by-line.

- 1. Title: The title of the document that encapsulates its main theme.
- 2. Description: A brief overview of the content and objectives of the file.
- 3. Practice Problems: Exercises aimed at reinforcing the learning of buffer solutions.
What happens if I fail to submit this form?
Failure to submit this form may result in lost opportunities to grasp complex concepts. It can hinder progress in understanding buffer solutions and their applications.
- Understanding Buffer Solutions: Not submitting could lead to gaps in knowledge about important chemical principles.
- Assessment and Grading: Incomplete submissions can adversely affect academic performance.
- Practical Application: Without submission, hands-on practice with buffer solutions may be missed.
How do I know when to use this form?

- 1. Laboratory Work: Utilize the form as a guide during experiments involving buffer solutions.
- 2. Classroom Learning: Use it as a resource during lessons focused on acid-base chemistry.
- 3. Self-Study: Refer to the form for independent learning and practice.
Frequently Asked Questions
What is a buffer solution?
A buffer solution is designed to resist pH changes when small amounts of acid or base are added.
How can I create a buffer solution?
You can create a buffer by mixing a weak acid with its conjugate base or vice versa.
What happens when I exceed a buffer's capacity?
Exceeding the buffer's capacity results in significant pH changes.
Can I use any acid or base to create a buffer?
Only weak acids and bases can form effective buffer solutions.
How do buffer solutions work?
Buffer solutions maintain pH by neutralizing added acids or bases using their acid-base pairs.
What is buffer capacity?
Buffer capacity is the limit to which a buffer can resist changes in pH.
What practical applications do buffer solutions have?
Buffer solutions are used in biological systems, laboratories, and industry to maintain stable pH levels.
How do I check if my buffer is effective?
The effectiveness of a buffer can be tested by adding known quantities of acid or base and observing pH changes.
Can I edit the provided PDF file?
Yes, you can edit the PDF using PrintFriendly's editing tools to add notes or make adjustments.
What formats can I download the edited PDF in?
You can download your edited PDF in standard formats suitable for printing and sharing.
Related Documents - Buffer Solutions Guide

Understanding Form and Form-Perception by James J. Gibson
This document explores various definitions and theories of form, emphasizing the need for precise terminology. It delves into experiments related to the visual perception of form, distinguishing between solid and surface forms. The text critiques traditional views and presents new perspectives on form-perception.

Prehistory of Peer Review: Religious Blueprints
This file explores the origins and development of peer review in science, tracing its roots to religious scholars in the Hartlib circle. It discusses the influence of the Royal Society of London and other early scientific organizations. The content is based on extensive historical research and analysis.

Analysis of Uncertainties in Radioactivity Measurements
This document discusses the uncertainties in analytical radioactivity measurements of environmental samples. It includes detailed equations and methods for calculating total propagated uncertainties. Useful for quality assurance specialists, data validators, and radiochemical analysts.

IRMS Sample Analysis Request Form Guidelines
This file contains instructions and details about the IRMS Sample Analysis Request Form. It is used to request sample analysis in the Laboratory for Isotopes and Metals in the Environment. Ensure you have the required approvals before using the IRMS.

Double Stuff Oreo Cookie Science Experiment
This file contains details and instructions for conducting a science experiment to evaluate the marketing claim of Double-Stuff Oreo cookies. Users will measure the mass of regular and Double-Stuff Oreo cookies along with their fillings. It guides users through the process of data collection, calculation, and analysis using the scientific method.
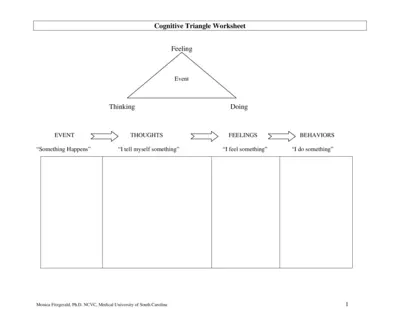
Cognitive Triangle Worksheet Instructions and Details
This file provides an overview and detailed instructions on how to use the Cognitive Triangle Worksheet. It helps users understand the relationship between their thoughts, feelings, and behaviors. Perfect for those interested in cognitive-behavioral strategies.

Engaging Doctor Pretend Play Printables for Kids
Transform playtime with free doctor pretend play printables designed for kids. These fun tools foster creativity and learning through imaginative play. Perfect for children from toddlers to first graders.

Ions and Ionic Compounds: Understanding Nomenclature
This file provides a comprehensive overview of ions, including their types, charges, and nomenclature rules. It covers essential details such as simple and polyatomic ions, and how to name them correctly. Perfect for students and professionals looking to deepen their understanding of ionic compounds.

Biology Form 3 Notes and Instructions
This file contains detailed biology notes for Form 3 students. It covers essential topics such as organism classification and characteristics of various kingdoms. Perfect for studying and exam preparation.
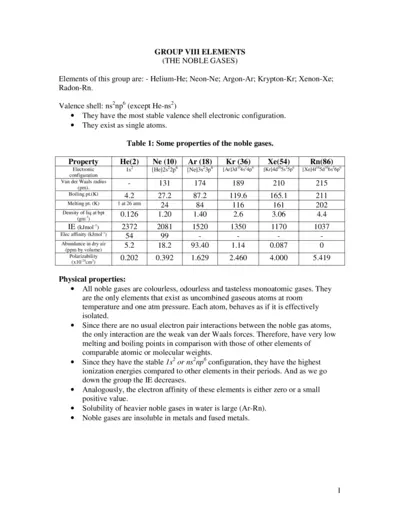
Noble Gases Properties and Chemical Behavior
This document provides a comprehensive overview of the noble gases, their properties, and chemical behaviors. It includes information on individual gases, their electronic configurations, and compound formations. Ideal for students and professionals in chemistry.

Chemical Bonding and Matter in Our Surroundings
This file provides detailed insights into chemical bonding, including ionic and covalent bonds. It covers the principles of matter in our surroundings and the electronic configurations of elements. Ideal for students and educators in chemistry to enhance their understanding.
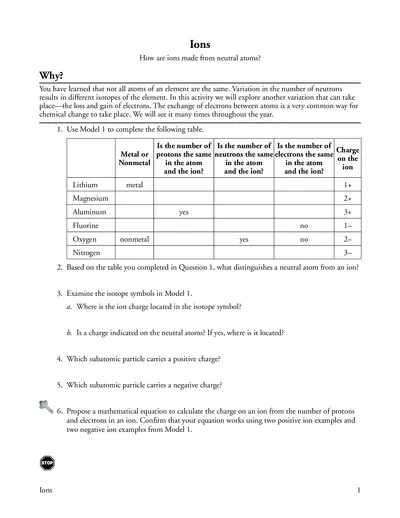
Understanding Ions and Their Formation in Chemistry
This file provides comprehensive insights into the formation of ions from neutral atoms. It covers the characteristics of cations and anions and explores the chemical changes involved. Perfect for students and educators seeking to understand atomic variations.