Edit, Download, and Sign the FDA Overview of Drug Manufacturing Inspections
Form
eSign
Add Annotation
Share Form
How do I fill this out?
To fill out this document, carefully read through each section to understand what information is required. Make sure you collect the necessary data before starting to fill in the form. Follow the prompts and ensure accuracy in your submissions for a successful outcome.
How to fill out the FDA Overview of Drug Manufacturing Inspections?
1
Review all sections of the document for understanding.
2
Collect all necessary information before filling out.
3
Begin filling the document with accurate information.
4
Double-check all entries for correctness and completeness.
5
Submit the form through the appropriate method outlined.
Who needs the FDA Overview of Drug Manufacturing Inspections?
1
Pharmaceutical companies need this file to comply with FDA regulations.
2
Quality assurance professionals reference this file to ensure standards are met.
3
Manufacturing plants require this file to document inspection results.
4
Regulatory affairs specialists use this file to understand FDA guidelines.
5
Healthcare providers may need this documentation for patient safety assurance.
How PrintFriendly Works
At PrintFriendly.com, you can edit, sign, share, and download the FDA Overview of Drug Manufacturing Inspections along with hundreds of thousands of other documents. Our platform helps you seamlessly edit PDFs and other documents online. You can edit our large library of pre-existing files and upload your own documents. Managing PDFs has never been easier.
Edit your FDA Overview of Drug Manufacturing Inspections online.
Editing PDFs on PrintFriendly has never been easier! You can modify the content directly in the PDF file using our intuitive editing tools. Simply select the areas you want to change and make your edits seamlessly.
Add your legally-binding signature.
With PrintFriendly, signing PDFs is a breeze! You can easily add your signature to the document with a few clicks. Our platform allows you to ensure that your signed documents are ready for submission.
Share your form instantly.
Sharing PDFs on PrintFriendly is simple and efficient! Once you have edited your document, you can share it directly with others via email or social media. Our user-friendly interface ensures that your shared files maintain their quality.
How do I edit the FDA Overview of Drug Manufacturing Inspections online?
Editing PDFs on PrintFriendly has never been easier! You can modify the content directly in the PDF file using our intuitive editing tools. Simply select the areas you want to change and make your edits seamlessly.
1
Open the PDF file in PrintFriendly editor.
2
Select the text or area you wish to edit.
3
Make the necessary changes to the content.
4
Review your edits for accuracy.
5
Download or save the edited document.

What are the instructions for submitting this form?
Submit this form via email to fda-submissions@example.com, or by fax at (555) 123-4567. You can also use the online submission portal found on the FDA website. Please ensure all required fields are filled out accurately to avoid processing delays.
What are the important dates for this form in 2024 and 2025?
Important dates for this form include submission deadlines for filing and upcoming inspection dates in 2024 and 2025. Stay updated on any regulatory changes announced by the FDA that may affect submission timelines. Being aware of these dates will help ensure compliance with necessary inspections and documentation.

What is the purpose of this form?
The purpose of this form is to ensure compliance with FDA standards during drug manufacturing inspections. It provides essential guidelines for drug manufacturers to adhere to while maintaining quality and safety in their processes. This form helps in documenting inspections and any subsequent actions required by the FDA.

Tell me about this form and its components and fields line-by-line.

- 1. Inspection Date: The date when the inspection is conducted.
- 2. Facility Address: The address of the facility being inspected.
- 3. Inspection Results: Summary of findings from the inspection.
- 4. Corrective Actions: Actions to be taken in response to inspection findings.
What happens if I fail to submit this form?
Failing to submit this form can lead to regulatory penalties and delays in the manufacturing process. Non-compliance may result in increased scrutiny from the FDA. It is crucial to adhere to the submission deadlines to avoid any negative consequences.
- Regulatory Penalties: Failure to submit may lead to fines and legal issues.
- Increased Scrutiny: Non-submission can cause the FDA to increase oversight of operations.
- Production Delays: Missing submission deadlines may halt production until compliance is achieved.
How do I know when to use this form?

- 1. Pre-Inspection Preparation: Use this form to prepare for upcoming FDA inspections.
- 2. Regulatory Compliance: Ensure adherence to FDA standards with timely submissions.
- 3. Documentation Maintenance: Keep records up to date for future inspections.
Frequently Asked Questions
Can I edit the PDF directly?
Yes, you can easily edit the PDF directly in the PrintFriendly editor.
How do I download my edited file?
After editing, simply click the download button to save your changes.
Is there a limit to how much I can edit?
You can edit the document as much as needed without restrictions.
Can I share the PDF with colleagues?
Absolutely! You can share your PDF via email or social media.
Is there a size limit for uploads?
Currently, there is no specific size limit for PDF uploads.
How secure is my document when editing?
Your document is safe when editing through PrintFriendly; security measures are in place.
What types of files can I edit?
You can edit any PDF file using the PrintFriendly platform.
Can I revert back to the original file after editing?
Once you edit and save, the original file can't be restored; please keep a backup.
Is there a mobile version of PrintFriendly?
Yes, you can easily access and edit PDFs using mobile devices.
How do I contact support for issues?
For any issues, you can reach out through the contact form on our website.
Related Documents - FDA Drug Manufacturing Inspections
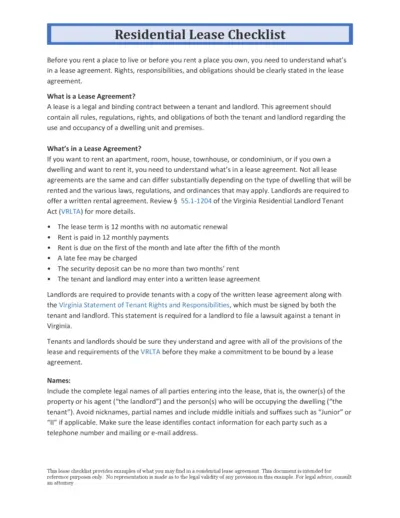
Residential Lease Agreement Checklist for Tenants and Landlords
This document provides a detailed checklist of what both tenants and landlords need to know and include in a residential lease agreement. It covers key elements such as lease terms, rent payment schedules, and maintenance responsibilities. Use this guide to ensure all rights and obligations are clearly outlined in your lease agreement.

Residential Lease or Month-to-Month Rental Agreement
This file contains a comprehensive residential lease or month-to-month rental agreement used in California. It provides details on terms, obligations, and conditions for both landlords and tenants. Perfect for those seeking a standardized rental agreement form.

Civil Court of the City of New York Nonpayment Petition
This document is a Notice of Nonpayment Petition issued by the Civil Court of the City of New York. It details the actions that a landlord can take against a tenant for nonpayment of rent. It includes instructions on how the tenant can respond and their rights.

Form 1099-MISC: Miscellaneous Income for 2013
This file is a 2013 version of the IRS Form 1099-MISC used to report miscellaneous income. It includes fields for reporting various types of payments made to individuals or entities. The form is typically filed by payers to report income paid to recipients.

Instructions for Form 706 (Rev. September 2023)
This document provides detailed instructions for completing Form 706, the United States Estate (and Generation-Skipping Transfer) Tax Return for decedents dying after December 31, 2022. It includes information on revisions, general instructions, and specific filing requirements. The instructions also cover important updates and reminders related to the form.

PhilHealth Report of Employee-Members Form Instructions
This file provides instructions for employers on how to fill out and submit the PhilHealth Report of Employee-Members form. It is essential for employers to report new hires to PhilHealth to ensure proper coverage. Detailed instructions and requirements are included.

Copyright Registration Form TX Instructions
This form is used for the registration of nondramatic literary works, such as fiction, nonfiction, poetry, textbooks, and computer programs. It provides detailed information on how to complete the form, including what information is required for each section and how to submit the application. Use it to ensure your work is properly registered for copyright protection.

Plaintiff's Claim and Instructions for Small Claims Court
This file provides instructions and necessary forms for filing a Plaintiff's Claim in Small Claims Court. It includes details on filling out, submitting, and serving the forms. Ensure to follow the steps carefully to protect your rights.

Ohio Sales and Use Tax Contractor's Exemption Certificate
This document is the Ohio Sales and Use Tax Contractor's Exemption Certificate. Contractors use this form to claim exemptions on certain taxable goods for specified exempt uses. It's crucial for contractors working with tax-exempt entities or on tax-exempt projects.

Lease Agreement for University of Florida Premises
This lease agreement file outlines the terms and conditions for renting a property owned by the Landlord to the University of Florida Board of Trustees. It covers key aspects such as lease term, rent details, improvements, and permitted use. Ideal for landlords and tenants involved in leasing agreements.

Return of Private Foundation Form 990-PF 2023
Form 990-PF is a return for private foundations required by the IRS. It includes information on revenue, expenses, and other financial details. Avoid entering social security numbers on this form.

Application Form for Divorce Certificate - Andhra Pradesh State Wakf Board
This form is used to apply for a Divorce Certificate from the Andhra Pradesh State Wakf Board in Hyderabad. The form requires details of both bride and groom as per recorded information. It also includes fields for verification and office use only.